In the rapidly evolving field of cancer treatment, antibody-drug conjugates (ADCs) are emerging as a powerful class of therapeutics against cancers. ADCs are complex molecules that combine the high specificity of monoclonal antibodies with the potent cytotoxic payloads, functioning like precision-guided “biological missiles” that target tumor cells while minimizing damage to healthy tissues.
ADC consists of three key components: an antibody, a linker, and a cytotoxic drug. The antibody serves as the “targeting system,” specifically recognizing antigens highly expressed on tumor cells (such as HER2 or CD30) with minimal expression on norma cells. The linker acts as a “bridge.” Stably connecting the antibody and the payload. It must remain stable in circulation to prevent premature release, yet cleave efficiently in the tumor microenvironment under acidic conditions or specific enzymatic activity to release the cytotoxic agent. The payload is the “warhead,” often a highly potent chemotherapeutic agent (eg, maytansinoid or auristatin), which disrupts critical processes such as DNA replication, protein synthesis, or cell division, ultimately inducing apoptosis (see Figure1) [1].
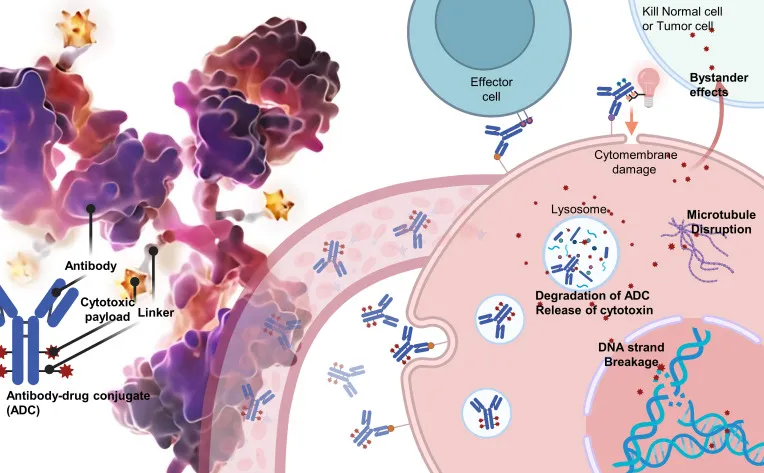
Figure 1: Structure and mechanism of action of an ADC[2]
This unique structure and targeted mechanism give ADCs significant potential in oncology, offering new hope for many cancer patients. However, fully leveraging their benefits requires precise monitoring of their pharmacokinetic (PK) behavior in vivo, which in turn demands highly sensitive and specific bioanalytical methods.
The Importance of ADC Bioanalysis
ADCs are inherently complex molecules, combining the properties of large-molecule biologics (antibodies) and small-molecule drugs (cytotoxic payloads). This hybrid nature presents multiple challenges for quantitative analysis. A comprehensive stability assessment of ADCs should include evaluation of the linker, payload, and mAb components, as well as the stability of the conjugate construct.[3]
Figure 2: Various forms of ADC-derived analysis in biological matrices
During drug development, accurate quantification of ADC concentration, drug-to-antibody ratio (DAR), and other critical quality attributes (CQAs) is essential to assess stability, ensure manufacturability, and guarantee batch-to-batch consistency. In the clinical setting, measuring ADC concentrations helps optimize dosing, predict efficacy, and identify potential adverse event, supporting personalized treatment approaches.
LBA Platform: High Specificity and Affinity
1. Principles and Advantages
Ligand binding assays (LBA) remain the platform of choice for quantifying total antibody (TAb) and conjugated ADC due to their high sensitivity, strong precision, and high sample throughput (see Figure 3). Common LBA platform include ELISA, MSD, and Gyros. Each platform offers distinct advantages and limitations. For instance, MSD and Gyros often provide higher sensitivity, broader dynamic range, lower sample volume requirements, and reduced matrix effects compared to traditional ELISA [4]. LBAs enable the development of high-throughput, cost-effective, and readily deployable ADC bioanalysis methods, which play a vital role in early-phase development and initial clinical sample analysis.
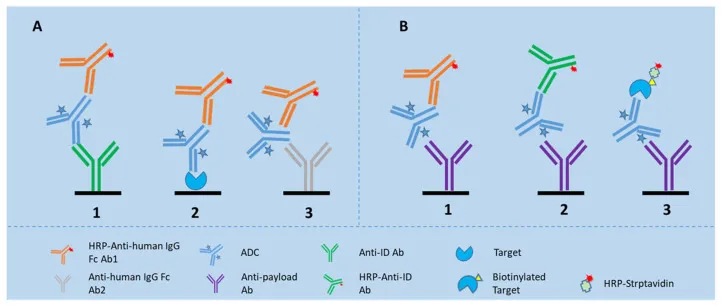
Figure 3: LBA-based detection of Tab and ADC in biological matrices
2. Applications in ADC Bioanalysis
In the field of ADC quantitative bioanalysis, LBA play an indispensable role. During early-phase ADC development, ELISA is commonly utilized for immunogenicity assessment. It detects whether patients development if the drug may induce an immune response that compromises efficacy. Furthermore, ELISA can measure the total concentration of ADC in biological samples, providing critical data to characterize its pharmacokinetic profile in vivo and supporting the determination of appropriate dosing and frequency.
LC-MS/MS platform: High Sensitivity and Precision
1. Principles and Advantages
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) combines the separation power of LC with the high sensitivity and specificity of MS/MS. It widely regards as the gold standard for small-molecule analysis and is commonly applied to quantify unconjugated payloads derived from ADCs.
Due to the high hydrophobicity of typical cytotoxic agents, released payloads are often extracted from biological matrices using protein precipitation (PP), solid-phase extraction (SPE), or liquid-liquid extraction (LLE), followed by LC-MS/MS analysis in multiple reaction monitoring (MRM) mode (see Figure 4). The platform also supports metabolite identification and profiling [5].
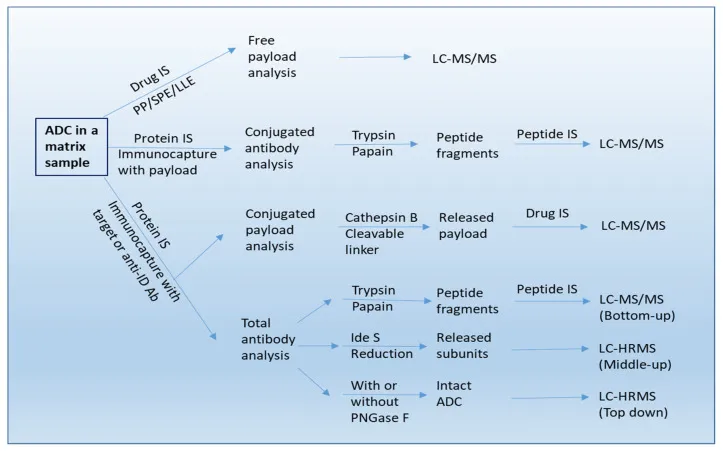
Figure 4: MRM mode in LC-MS/MS anslysis
LC-MS/MS offers exceptional sensitivity often surpassing LBA making it ideal for trace-level analyte detection. It enables accurate DAR determination, identifies drug-related degradation products, and provides rich structural data for understanding drug metabolism and mechanisms. Moreover, LC-MS/MS is highly versatile and can be adapted for various ADC formats and analytes.
2. Applications in ADC Bioanalysis
LC-MS/MS is considered the gold standard for assessing DAR during ADC manufacturing and quality control. It ensures that each batch meets predefined specifications for potency and consistency. In clinical studies, LC-MC/MS enables precise quantification of ADC payload and related metabolites in plasma, supporting PK/PD modeling and dose optimization. For example, in a clinical trial for advanced breast cancer, LC-MS/MS was used to monitor ADC concentrations in near real-time, allowing dose adjustments that improved efficacy and reduced adverse events.
Integrated Approaches
Given the complementary strengths and limitations of LBA and LC-MS/MS, many programs adopt a hybrid strategy. LBA is often used for high-throughput screening in early development, whereas LC-MS/MS delivers detailed structural and metabolic data in later stages. In clinical testing, LBA may serve as a first-line tool for sample screening, with LC-MS/MS used for confirmatory or specialized analysis. This integrated approach enhances both efficiency and data reliability.
As ADC therapeutics continue to evolve and enter broader clinical use, the requirements for quantitative bioanalysis will grow increasingly sophisticated. Both LBA and LC-MS/MS are indispensable tools in the ADC development toolkit. Future technological advances will further refine these platforms, delivering even greater precision and reliability to support ADC research, manufacturing, and clinical translation. This progress is pivotal in driving critical breakthroughs across the biopharmaceutical sector, ultimately bringing new hope to cancer patients worldwide.
Amador Bioscience (Hangzhou), a subsidiary of SDM Bioservices, operates state-of -the-art technology platforms, including plate readers, MSD, LC-MS, flow cytometers, and qPCR systems. It delivers a broad range of research and regulatory-grade laboratory services such as pharmacokinetics (PK), anti-drug antibody (ADA) and neutralizing antibody (NAb) assays, cytokine release testing, immunophenotyping, and pharmacodynamic biomarker analysis.
The company supports bioanalysis for diverse therapeutic modalities, such as monoclonal antibodies, bispecifics, antibody prodrugs, ADCs, fusion proteins, peptides, cell and gene therapies, oligonucleotides, and small molecules. Its comprehensive service portfolio covers PK analysis, immunophenotyping, ADCC, and ADC.
Located in Hangzhou, China, Amador’s laboratory is capable of supporting hundreds of bioanalysis and biomarker projects annually.
The Amador Hangzhou bioanalytical lab has particularly extensive experience in ADC-related analyses. It offers well-established capabilities using LBA, LC-MS/MS, and flow cytometry platforms for quantifying total antibody, conjugated antibody, conjugate drug, and free small molecule PK, as well as assessing ADC immunogenicity and biomarkers.
Notably, Amador Hangzhou has successfully developed a hybrid immune-capture LC-MS/MS platform for simultaneous determination of total antibody, conjugated antibody, conjugated drug, and free payload in ADCs. This approach reduces reliance on specific reagent antibodies, enables rapid method development and validation, and supports hig-throughput sample analysis, significantly accelerating project timelines and advancing drug development efforts.
References:
[1] Fu Z,Li S,Han S,et al.Antibody drug conjugate: the“biological missile” fortargeted cancer therapy. Signal Transduct Target Ther. 2022;7:93.
[2] Li K, Xie G, Deng X,et al. Antibody-drug conjugates in urinary tumors: clinical application, challenge, and perspectives. Front Oncol. 2023 Dec 20;13:1259784.
[3] Cahuzac H, Devel L. Analytical Methods for the Detection and Quantification of ADCs in Biological Matrices. Pharmaceuticals (Basel). 2020 Dec 14;13(12):462.
[4] Myler H., Rangan V.S., Wang J., et al. An integrated multiplatform bioanalytical strategy for antibody-drug conjugates: A novel case study. Bioanalysis. 2015;7:1569–1582.
[5] Kaur S., Xu K., Saad O.M., Dere R.C., Carrasco-Triguero M. Bioanalytical assay strategies for the development of antibody-drug conjugate biotherapeutics. Bioanalysis. 2013;5:201–226.
This session focuses on Stakeholder Engagement: developing strategies for collaborative project decision-making and execution.
The updated draft guidance involves a focus on clinical trial design, regulatory considerations, and whether these trials can demonstrate that the drugs can maintain weight loss as determined by BMI.
Let's take a look back at the previous two installments of the Clinical Data Management “PM” series: Scope Management, which clarifies the scope of responsibilities of all parties involved in a clinical trial, and Project Resource Management, which focuses on the utilization of company and personal resources to accomplish data management tasks. In this installment, we will focus on the most important part of project management - project schedule management - to share the timeline planning and progress follow-up of data management activities, so as to efficiently complete the data management work under the premise of ensuring the data quality and reaching the important milestones of the project.
In the last session, we learned about project scope management for data management work, identifying the scope of responsibilities for data cleansing and data management activities. After defining the scope of the data management work, in this session, we will learn how to mobilize the resources within the scope of work to carry out the data management work more efficiently and with higher quality.
Shanghai SDM Vaccine Data Management Department, in collaboration with the International Project Department, has launched a series of training sessions on "Application of Project Management Knowledge in Data Management." The Application of Project Management Knowledge in Data Management Work contains eight modules, including Project Integration Management, Project Scope Management, Project Progress Management, Project Quality Management, Project Resource Management, Project Communication Management, Project Risk Management, and Project Stakeholder Management, etc. It mainly refers to the theoretical knowledge of the Guide to the Project Management Body of Knowledge (PMBOK Guide) and combines the content of the data management work and practical experience of the project.
Want to quickly penetrate the Chinese, American and European pharmaceutical markets? Registering for communication exchanges is the key!
SDM PV team detects drug safety risks.
SDM Vaccine Experts Share Roadmap to Avoid Clinical Trial Pitfalls.
this article outlines essential documentation preparation and strategic considerations for conducting pre-IND communication meetings with CDE, ensuring effective regulatory alignment and adequate guidance.
Global Vaccine Solutions via Multidimensional Strategies.
July 23, 2025 GSK disclosed that the FDA has postponed PDUFA date of the Blenrep® (belantamab mafodotin) combination therapy BLA. The agency established a new action date of October 23, 2025 for completion of BLA review.
SDM Bioservices has successfully established a hybrid immuno-capture LC-MS/MS platform for the simultaneous quantification of ADC total antibody, conjugated antibody, conjugated drug, and free small-molecule payload. This approach significantly reduces reliance on specific antibody reagents, enables rapid method development and validation, and supports high-throughput sample analysis—thereby accelerating project timelines and advancing drug development efforts.
Get in touch with SDM experts for your questions or comments and a member of our team will get back to you directly.
Let's Start a Conversation