In the previous sessions of the clinical data management “PM” training series, we covered four key topics:
1. Scope Management: Defining stakeholder roles
2. Resource Management: Optimizing task execution
3. Schedule Management: Milestone tracking
4. Quality Assurance: Deliverable validation
This session focuses on Stakeholder Engagement: developing strategies for collaborative project decision-making and execution.
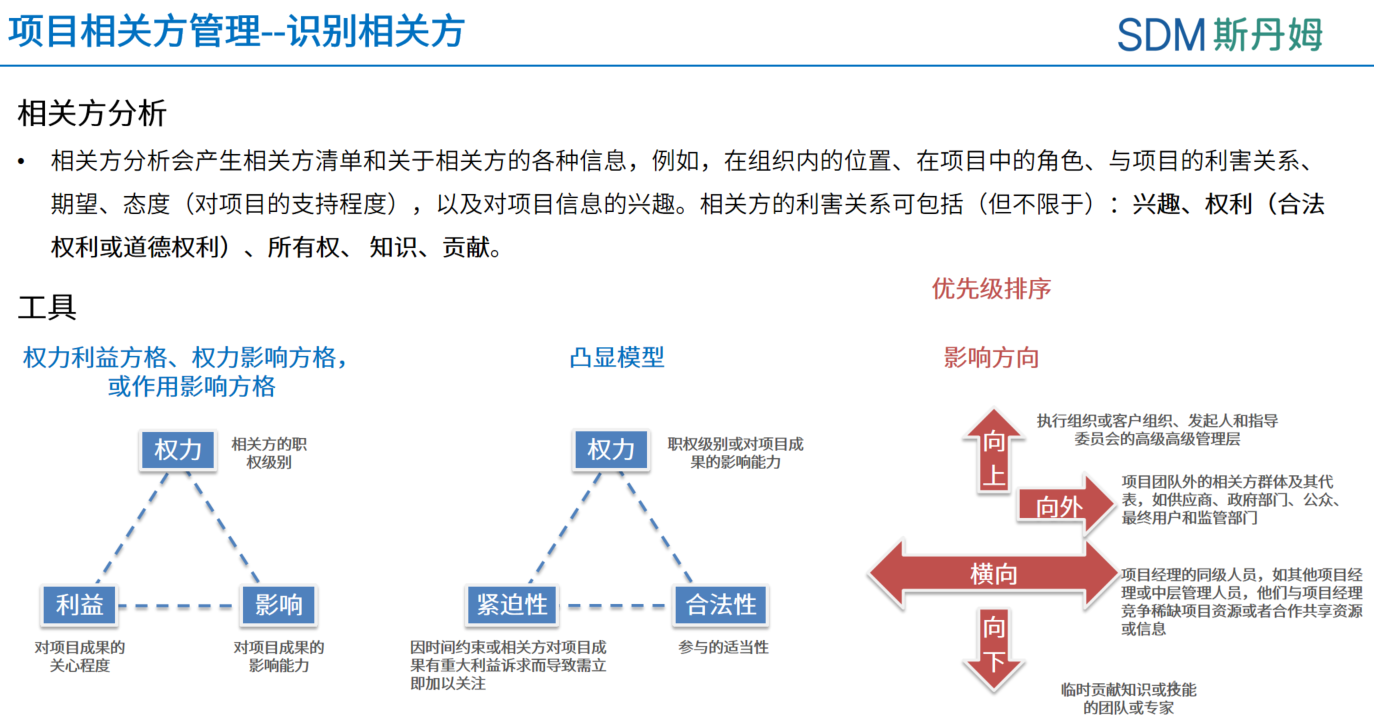
Stakeholder management involves: Identification-Mapping key parties impacted by/influencing projects, analysis-Assessing expectations and impact levels, strategy-Developing engagement frameworks for decision-making and execution-Implementing & adjusting communication plans.
Every project involves stakeholders who can either support or hinder its progress, with varying levels of influence. Some have minimal impact, while others significantly shape outcomes. When challenges emerge, start by identifying key stakeholders, understanding their expectations and perspectives, then adapt communication and resolution tactics to align with their priorities and influence.
In the various tasks related to data management
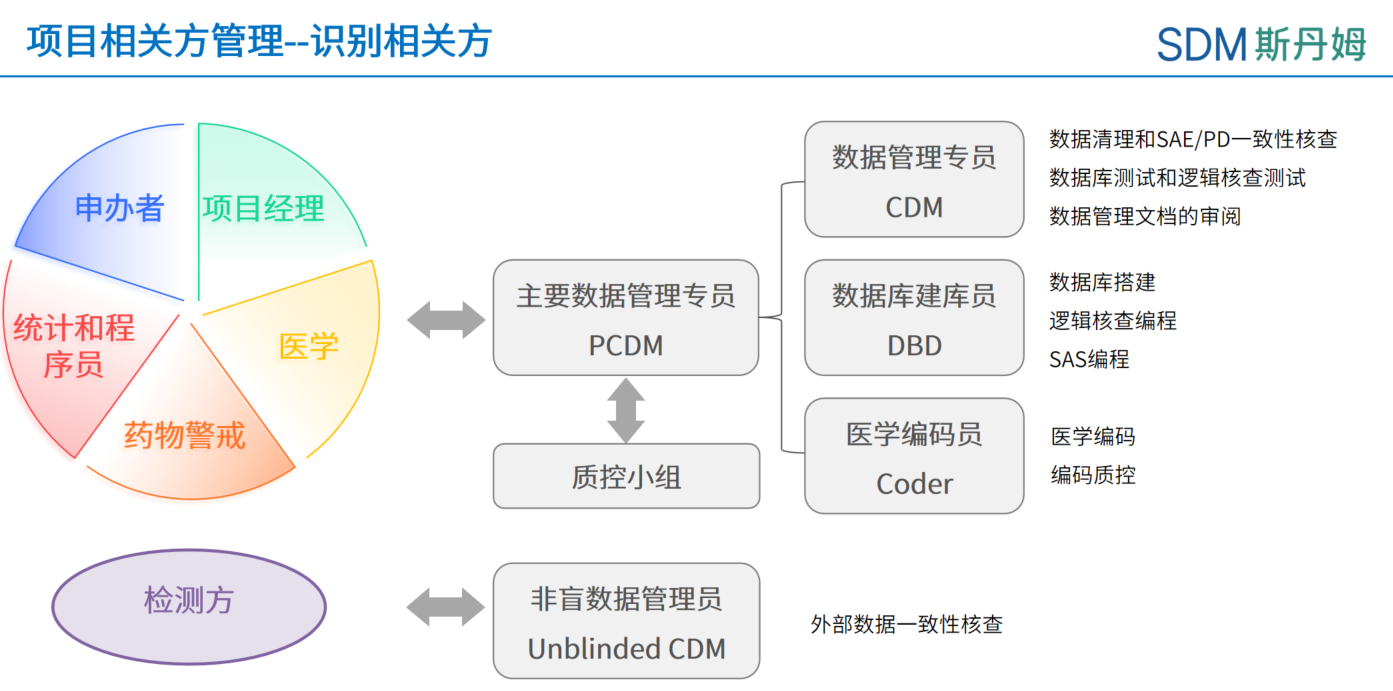
Primary Clinical Data Manager orchestrates stakeholder collaboration through three dimensions:
Cross-functional Coordination: Interfaces with project managers, medical monitors, pharmacovigilance, biostatisticians, and programmers for data alignment.
Internal Workflow: Leads data management teams (blind/non-blind DMs, database developers, medical coders) with SME consultation.
External Integration: Manages vendors (EDC/ePRO systems, external data) and sponsor communications.
Escalation Protocol: Activates managerial intervention (line managers/domain leads) for high-risk issues requiring technical/resource mobilization.
As the saying goes, “Know yourself and know your enemy, and you will never lose a battle.”
In data management, effective collaboration with stakeholders like the Project Manager is critical. The PM oversees tasks from project preparation to closure, including scheduling, cross-team coordination, and ensuring timely progress. During initiation, the PCDM and PM must align on key details such as the first patient enrollment timeline, EDC/randomization system vendor selection, payment methods, and medical coding dictionary copyright ownership. For projects with multiple communication channels, they should clarify protocols: whether data-related tasks (document review, database testing, data cleaning updates) will be handled directly between the PCDM and the sponsor’s data team or routed through the PM, and whether investigator communications will be managed by the site’s LCRA or the PM. Ensuring clarity in these areas enhances cross-team collaboration efficiency.
During project implementation, the PCDM coordinates with the PM to align data cleaning schedules with site enrollment and data entry plans. Regular updates on key metrics (e.g., SDV rates, data entry progress, query resolution status) and data-related risks should be shared with the project team. For significant data verification issues requiring bulk queries, the PCDM should notify the PM in advance to allow coordination with CRAs for targeted site training, minimizing redundant queries and reducing the burden on researchers.
During protocol updates, the PM must promptly notify the team of the revised version and effective date, coordinating protocol training with the medical team if required. The PCDM should evaluate whether data documents or the database need adjustments and plan updates (timeline/content) accordingly. Notably, project meetings are not solely PM-driven. The PCDM can also initiate discussions for data-related issues to align stakeholders.

Project Manager profiles
In data management, the PCDM team collaborates closely with Medical Writers and Medical Monitors. Medical Writers draft key trial documents like protocols, informed consent forms, case report forms, and clinical study reports. Medical Monitors review data for safety, eligibility criteria, and trial compliance, offering guidance on drug safety and trial processes. This partnership ensures alignment between data management and medical oversight throughout the project.
During the project lifecycle, the Medical Monitor reviews key data documents (e.g., CRFs, DMP, DVP, eCCI) in the initiation phase and participates in medical coding, protocol deviation reviews, and EDC data validation during implementation. For data issues requiring medical expertise, the PCDM collaborates with the Medical Monitor and teams to establish data entry rules, evaluate impacts on statistical outcomes and the CSR report, and document agreed-upon resolutions.

Medical Manager profiles
In data management, the PCDM collaborates closely with statisticians to ensure accurate, analysis-ready data. During project initiation, statisticians review CRFs to confirm all necessary data points are captured and provide randomization parameters (e.g., blinding rules, statistical guidelines) if applicable. Throughout implementation, they advise on data issues from a statistical perspective. Post-database lock, the PCDM promptly delivers EDC and external datasets to statisticians for generating analytical outputs, maintaining alignment between data quality and statistical requirements.
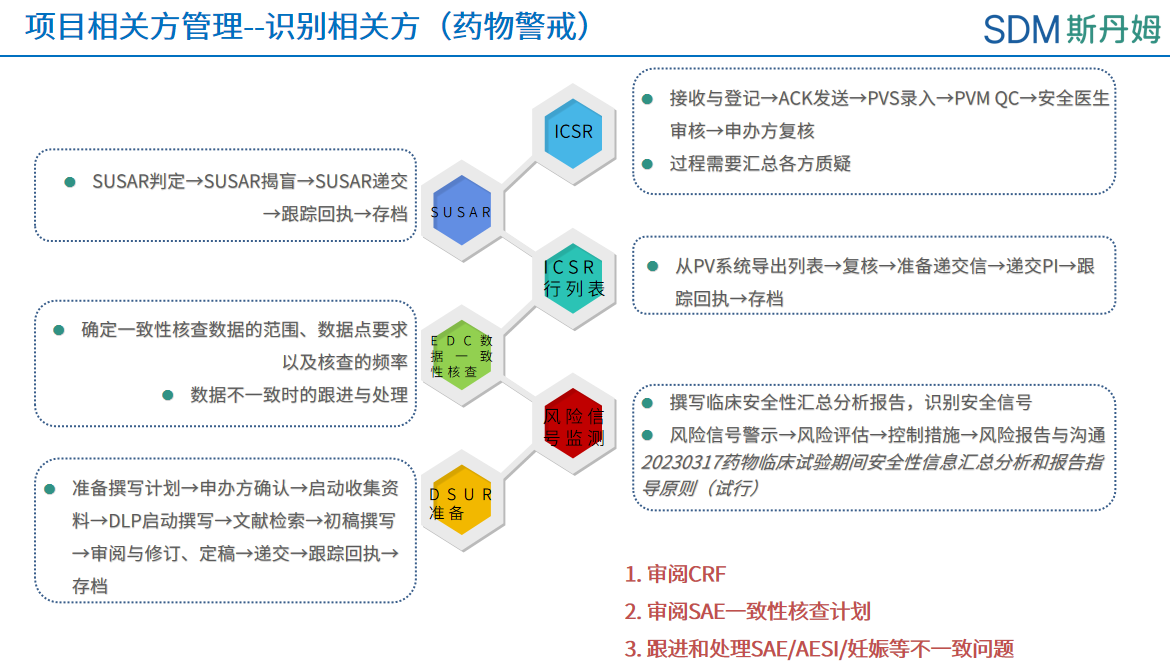
The Pharmacovigilance team handles safety reporting (e.g., ICSRs, SUSARs) and risk monitoring. PV collaborates with data management (PCDM) to align SAE data between the EDC and PV systems. During project initiation, PV reviews the CRF and SAE consistency check plan. In implementation, PV and PCDM resolve SAE discrepancies to ensure all issues are closed before database lock, maintaining data integrity across both systems.
Pharmacovigilance profiles
In data management, the sponsor (as the ultimate owner of data quality) and line managers are key stakeholders for PCDM. Sponsors should engage early with PCDM to clarify requirements, ensuring alignment in planning and avoiding misaligned assumptions. During implementation, PCDM updates sponsors on progress and seeks input on critical decisions (e.g., EDC system selection, coding dictionary licensing), while sponsors review documents, test databases, and confirm priorities per agreed terms. PCDM should prioritize proactive guidance over open-ended requests to sponsors, minimizing scenarios requiring sponsor judgment.
For unresolved challenges or communication barriers, PCDM should escalate to line managers promptly to prevent delays. Line managers should participate in meetings, monitor communications, and provide targeted support to resolve issues efficiently.
Stakeholder Engagement in Data Management:
Effective stakeholder management requires understanding their needs, expectations, and potential project impact. Stakeholders may fall into categories ranging from unaware to resistant, neutral, supportive, or leading. When facing resistance, PCDM should first analyze the issue’s context, empathize with stakeholder perspectives, and adapt communication strategies to align interests. Avoid rigidly defending positions or prioritizing personal goals, as this risks stalemate. Proactive, solution-focused dialogue ensures smoother collaboration and resolution.

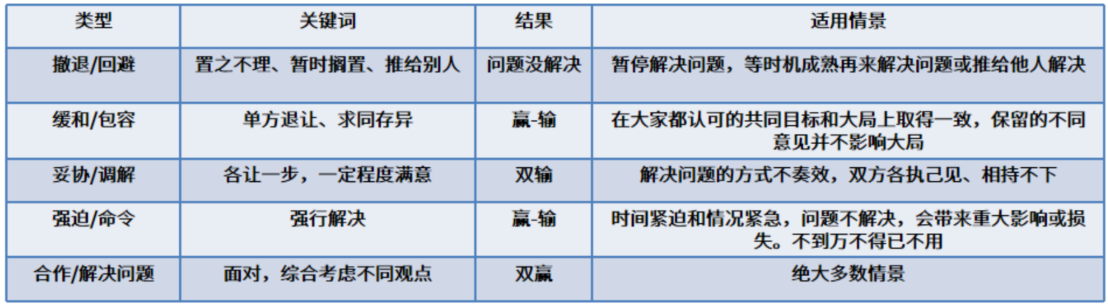
Stakeholder Engagement & Conflict Resolution: Stakeholder engagement involves proactive communication and collaboration to align needs, resolve issues, and foster constructive participation. Conflicting priorities or perspectives among stakeholders are inevitable.
Common resolution strategies include avoidance, accommodation, compromise, enforcement, and collaborative problem-solving, each offering guidance to navigate disagreements effectively.
In clinical trials, designing the Case Report Form involves balancing diverse stakeholder priorities. Project Managers prioritize aligning the CRF with investigator workflows and CRA review processes to simplify data entry and source verification. Medical Monitors advocate for granular adverse event details (e.g., daily symptoms, allergic reactions) to enhance safety oversight, though this increases data collection and validation burdens. Statisticians, however, emphasize CDASH-standardized data structures to streamline programming and analysis. This interplay highlights the challenge of reconciling operational feasibility, safety rigor, and analytical efficiency in CRF design.
In CRF design discussions, the PCDM should pre-assess stakeholder priorities and potential conflicts (e.g., via Table 1) to align recommendations with industry standards and project needs. Balancing perspectives, without favoring one stakeholder, prevents operational inefficiencies or risks.
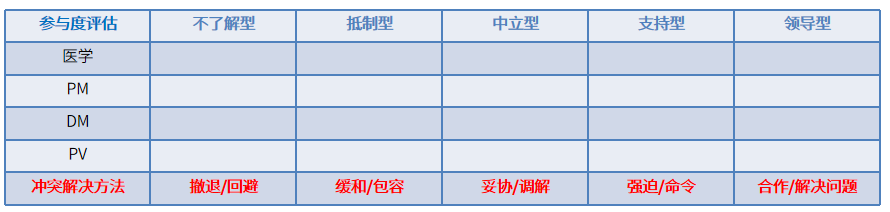
Table 1 Clinical trial stakeholder participation evaluation table
Successful projects hinge on proactive stakeholder engagement: understanding expectations, resolving conflicts strategically, and guiding collaborative decisions to optimize outcomes.
This session focuses on Stakeholder Engagement: developing strategies for collaborative project decision-making and execution.
The updated draft guidance involves a focus on clinical trial design, regulatory considerations, and whether these trials can demonstrate that the drugs can maintain weight loss as determined by BMI.
Let's take a look back at the previous two installments of the Clinical Data Management “PM” series: Scope Management, which clarifies the scope of responsibilities of all parties involved in a clinical trial, and Project Resource Management, which focuses on the utilization of company and personal resources to accomplish data management tasks. In this installment, we will focus on the most important part of project management - project schedule management - to share the timeline planning and progress follow-up of data management activities, so as to efficiently complete the data management work under the premise of ensuring the data quality and reaching the important milestones of the project.
In the last session, we learned about project scope management for data management work, identifying the scope of responsibilities for data cleansing and data management activities. After defining the scope of the data management work, in this session, we will learn how to mobilize the resources within the scope of work to carry out the data management work more efficiently and with higher quality.
Shanghai SDM Vaccine Data Management Department, in collaboration with the International Project Department, has launched a series of training sessions on "Application of Project Management Knowledge in Data Management." The Application of Project Management Knowledge in Data Management Work contains eight modules, including Project Integration Management, Project Scope Management, Project Progress Management, Project Quality Management, Project Resource Management, Project Communication Management, Project Risk Management, and Project Stakeholder Management, etc. It mainly refers to the theoretical knowledge of the Guide to the Project Management Body of Knowledge (PMBOK Guide) and combines the content of the data management work and practical experience of the project.
Want to quickly penetrate the Chinese, American and European pharmaceutical markets? Registering for communication exchanges is the key!
SDM PV team detects drug safety risks.
SDM Vaccine Experts Share Roadmap to Avoid Clinical Trial Pitfalls.
this article outlines essential documentation preparation and strategic considerations for conducting pre-IND communication meetings with CDE, ensuring effective regulatory alignment and adequate guidance.
Global Vaccine Solutions via Multidimensional Strategies.
July 23, 2025 GSK disclosed that the FDA has postponed PDUFA date of the Blenrep® (belantamab mafodotin) combination therapy BLA. The agency established a new action date of October 23, 2025 for completion of BLA review.
SDM Bioservices has successfully established a hybrid immuno-capture LC-MS/MS platform for the simultaneous quantification of ADC total antibody, conjugated antibody, conjugated drug, and free small-molecule payload. This approach significantly reduces reliance on specific antibody reagents, enables rapid method development and validation, and supports high-throughput sample analysis—thereby accelerating project timelines and advancing drug development efforts.
Premier Li Qiang has signed a State Council decree, promulgating the "Regulations on the Administration of Clinical Research and Translation of Novel Biomedical Technologies." This important regulation was adopted at the State Council executive meeting on September 12, 2025, and will take effect on May 1, 2026. This establishes a comprehensive legal framework for China's oversight of novel biomedical technologies throughout the entire chain from research to application.
Get in touch with SDM experts for your questions or comments and a member of our team will get back to you directly.
Let's Start a Conversation