Let's review the Clinical Data Management “PM”series that we shared in previous two sessions: Scope which clarifies responsibilities of all parties involved in a clinical trial and Project Resource Management which focuses on the utilization of company and personal resources to accomplish data management tasks. In this session, we will focus on project schedule management which is the most important aspect of project management to share the timeline planning and follow-up of data management activities so to efficiently complete the data management work under the premise of ensuring data quality and reaching important project milestones.
Project schedule management includes all the processes needed to ensure that the project is completed on time, including 6 major processes: planning schedule management, defining activities, scheduling activities, estimating time duration, developing schedule plans and controlling progress. “Preparedness ensures success, unpreparedness spells failure”, which develops the progress plan is a particularly important part of project management. The schedule plan is used to provide a detailed plan of how and when the project will deliver the products, services, and outcomes defined in the project scope, and is a tool used to communicate and manage the expectations of interested parties, providing a basis for performance reporting. Where possible, the flexibility of the detailed project schedule should be maintained throughout the duration of the project so that it can be adjusted as knowledge is gained, risks are better understood, and value-added activities are designed [1].
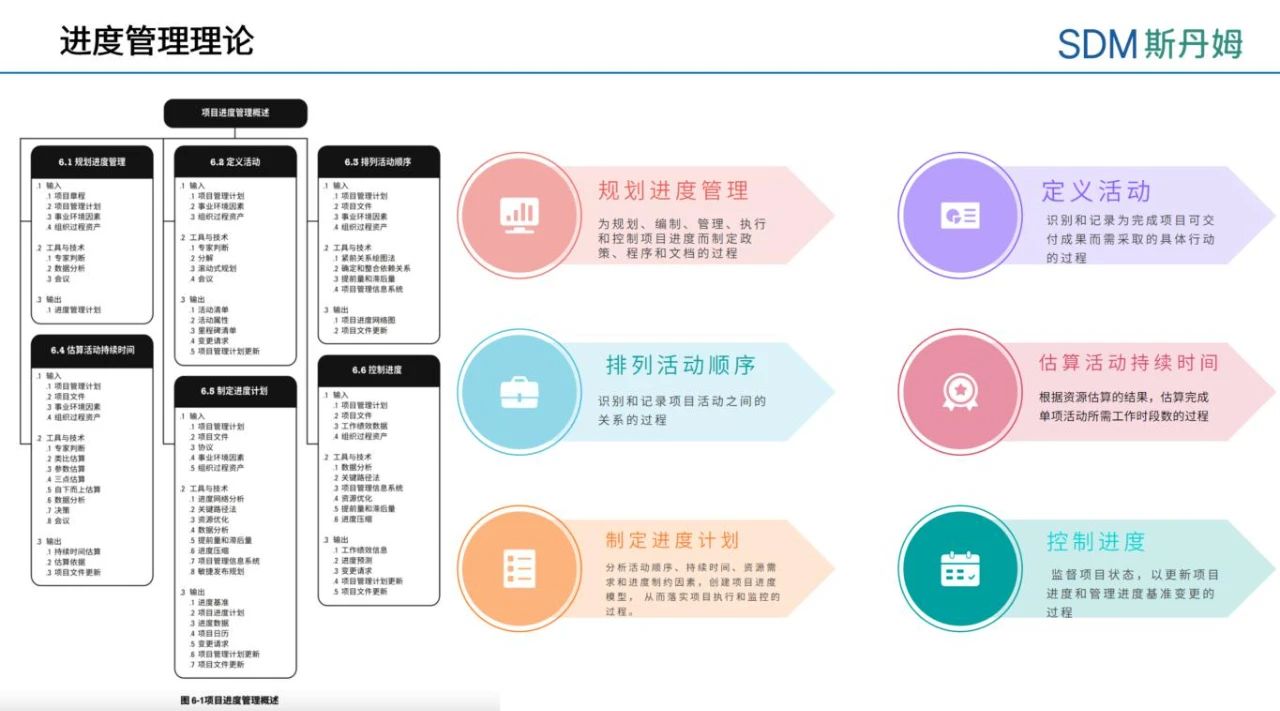
For clinical data management activities, developing a timeline plan is equally critical. Next, we'll cover the key considerations for developing a timeline plan and following up on project progress in terms of the three phases of data management activities: project initiation, cleansing, and database locking.
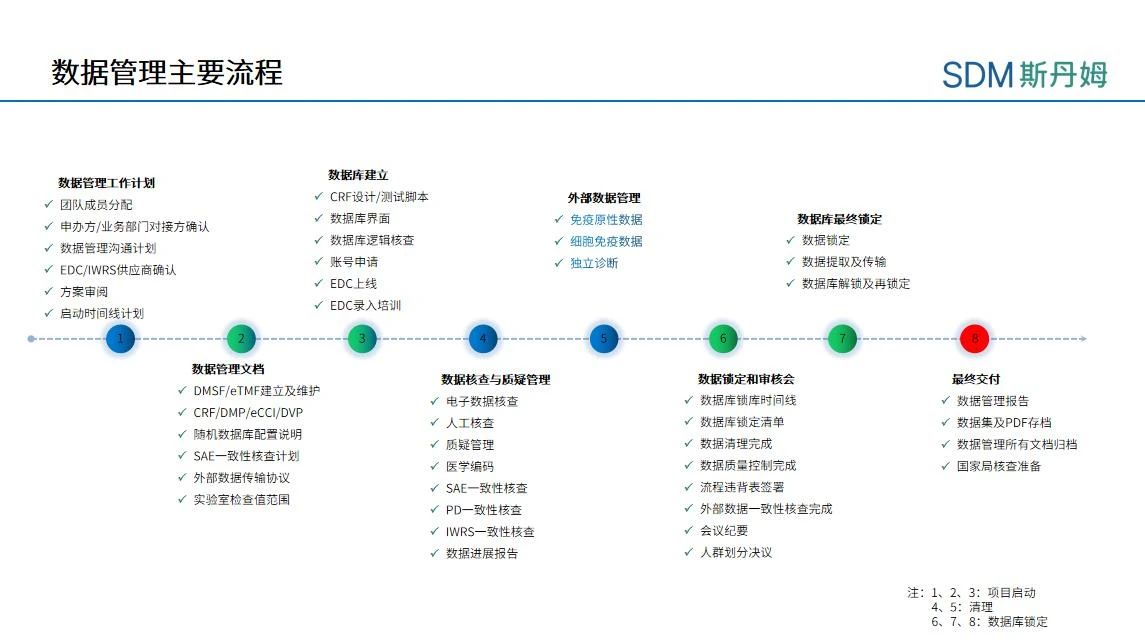
Key processes for data management (using the vaccine program as an example)
Progress management in the data management start-up phase
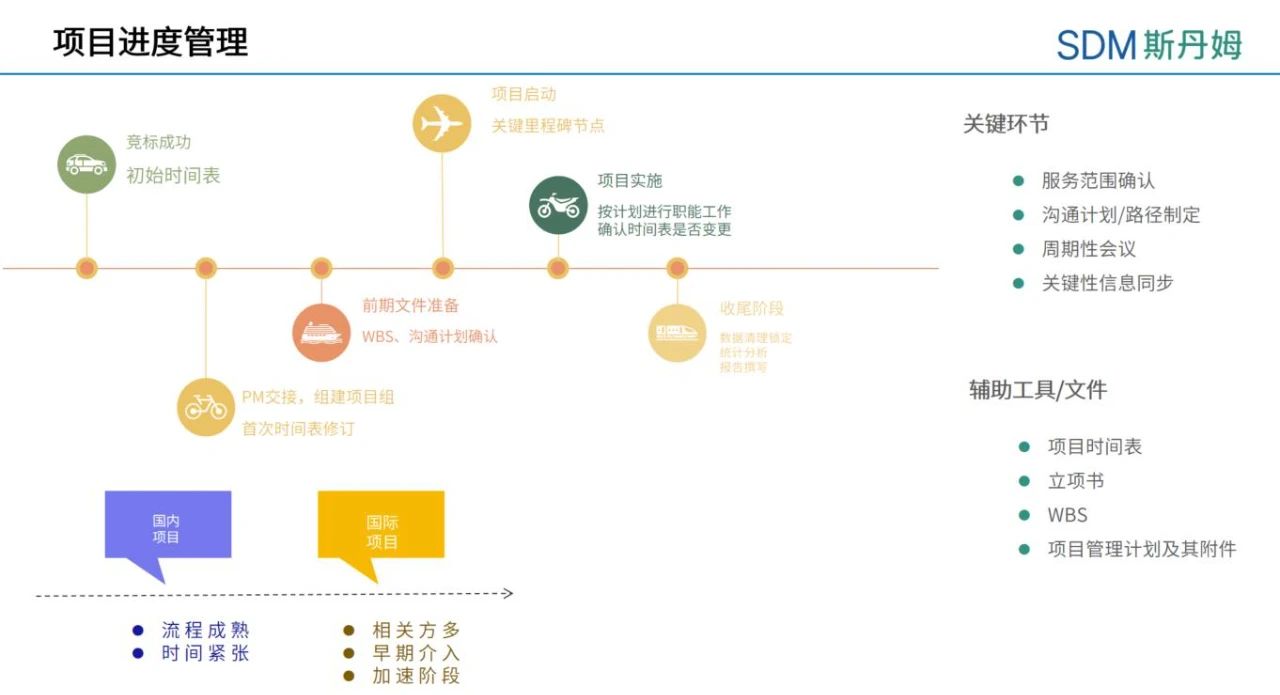
In the early preparation phase of the project, the data management team can start to intervene in the clinical trial related work. In addition to reviewing the protocol, forming the team, and confirming the EDC/IWRS system selection, they should also give an analysis of the major difficulties and solutions of the data management work in response to the project requirements, provide feedback on the timeline schedule of the data management activities based on the initial Project Timetable formulated by the project manager, as well as prepare a Data Management Communication Plan for the project. The initial Project Timeline needs to be adjusted according to the project requirements and actual project progress during the subsequent project progress, e.g., for data management activities, the selection of EDC will affect the timeline schedule of database construction. If the project intends to activate the new EDC, it is necessary to confirm the database builder, sort out the system function settings, and arrange corresponding training in advance. To familiarize with the operation of the new system, more time needs to be reserved for the project team for User Acceptance Testing (UAT).
Team should pay particular attention to the impact of system account management and workflow settings on the project and consider adding new document templates if necessary. If the project uses a randomization system to randomize and dispense medications, you should also check with the project manager or supply management manager in advance to confirm the lead time for the medication supply plan, and you should also clarify which parties are responsible for each role in the randomization system, paying particular attention to the configuration of blind and non-blind roles.
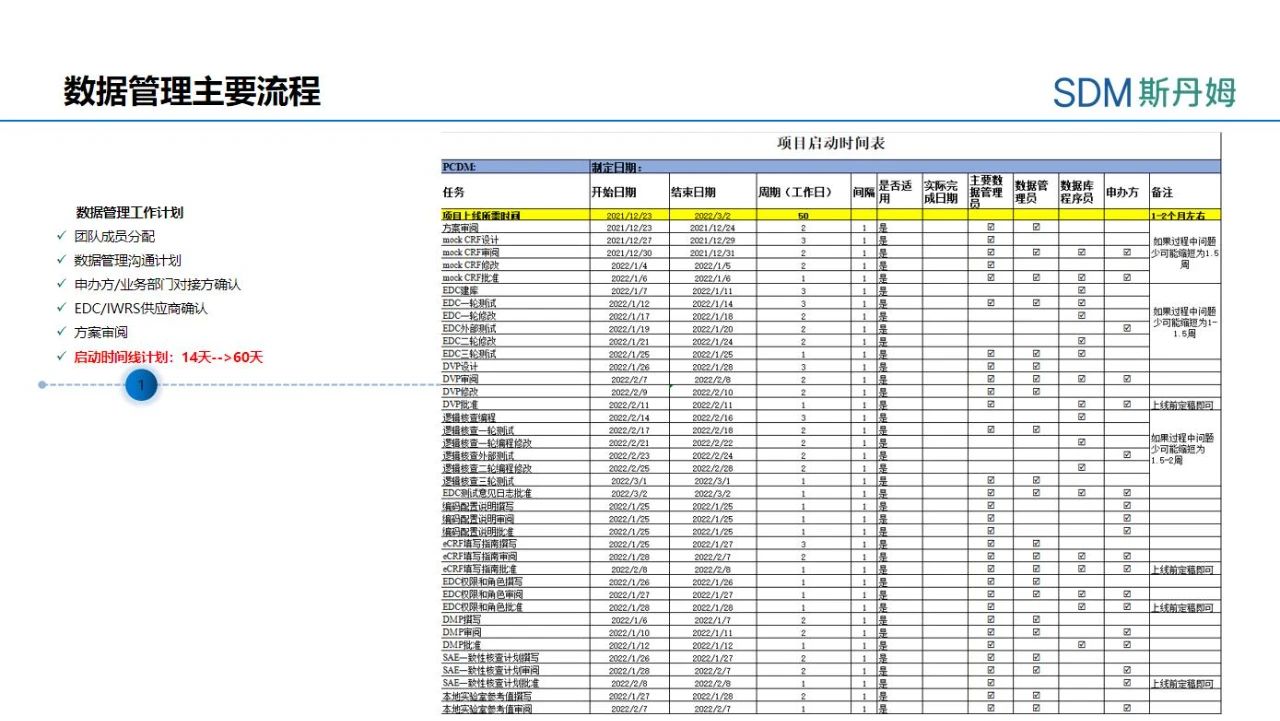
After determining the basic information of the project, the Primary Clinical Data Manager (PCDM) needs to develop a detailed project start-up schedule for the data management activities in the start-up phase, and needs to specify the start and end time of each activity in the schedule, and the support needed from all parties. Data management start-up often takes 14 to 60 business days, and the PCDM needs to schedule activities according to the needs of the project (e.g., enrollment of the first subject), and the actual situation (e.g., functionality of the EDC/randomization system, staffing, etc.). The opening of some data management activities will have a clear sequence, a stable version of the program is the first condition to open the data management activities, the PCDM needs to write the Case Report Form (CRF) according to the program, the vaccine project should also be combined with some of the original data such as follow-up book, diary card design to adjust the order of the CRF, in order to facilitate the field researchers or CRC than the original data. When the CRF has been reviewed and stabilized by multiple parties, the writing of the Data Validation Plan (DVP) can begin. However, some data management activities can go hand in hand, such as DVP and electronic Case Report Form Completion Instruction (eCCI) can be written in parallel. For projects with very urgent timelines, the strategy of going live with the interface before the first subject is enrolled and then going live with the logic verification can also be used to achieve the condition that data entry can be performed at the time of the first subject's enrollment.
Impact factors for pre-launch work
For the whole project, the key milestone event in the start-up phase is the first subject enrollment, and the EDC system needs to complete the go-live work before the first subject enrollment. Taking vaccine trial as an example, rate limiting steps in the start-up phase usually include obtaining clinical trial approval, vaccine validation, protocol finalization, site evaluation and assessment, contract signing for the research center, ethical approval, etc. The CRF writing and database building will start from the protocol finalization, which is usually not a speed limit step affecting the project operation, and the data management team will complete the go-live work of the EDC/IWRS system before the enrollment of the first subject, which is sufficient. However, in order to better coordinate project resources, as data managers we can also learn more about operationally related factors that affect project startup to better schedule the timeline of data management activities. For example, some EDC systems start billing only after going live, PCDM can rationally plan the system go-live time according to the overall progress of the project in order to minimize the additional costs incurred.
Administrative processes usually take a long time prior to the initiation of clinical trials, and the influencing factors on the progress of common projects before initiation are shown in Table 1, such as the application for IND and the communication between the sponsor and the Center for Drug Evaluation (CDE) of the State Drug Administration; such as ethical approvals, and the clinical trials usually carried out in hospitals need to be confirmed in advance for the ethical meeting frequency and schedule, the overall cycle of the ethical process of the establishment of the project is longer than the time cycle of obtaining ethical approvals from the CDE, and projects involving the filing of the Human Genetic Resource Administration of China also need to obtain ethical approvals and the signing of contracts by all parties before they can be filed.
For overseas clinical trials, due to the different situations in each country, obtaining INDs is also a major speed-limiting step, and sometimes the time from application to approval is longer, but once the approval is obtained, the entire project can enter an accelerated preparation phase, where the preparation of data documents and database building tests need to be quickly initiated and completed. Unlike domestic vaccine clinical trials, the timeline for overseas trials can also be affected by a variety of factors, such as the export of vaccines and other supplies, transportation, customs clearance, flight status, and investigator cooperation.
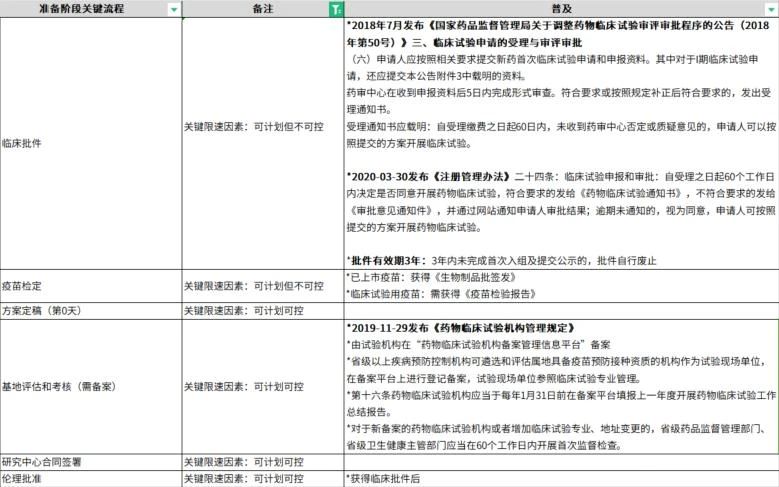
Table 1: Influences on common project schedules prior to start-up
After the database goes live, the data manager is required to perform various data management activities at the frequency specified in the Data Management Plan (DMP, Data Management Plan): data cleanup, challenge management, medical coding, serious adverse event consistency verification, protocol deviation processing, external data consistency verification, quality control, data progress report writing, etc., and the pace of the work and the tasks will be relatively smooth. During the smooth period of the project, PCDM needs to monitor and follow up issues related to the progress of data management in the following three areas:
1. It is recommended to follow up on the project's key data performance indicators at a monthly frequency, such as the SDV ratio, page entry rate, average length of time between the occurrence of a visit and data entry, query response rate, one-time closure of the query rate, and the number of SAE consistency verification issues, etc., and to communicate with the project team in a timely manner on the performance indicators that appear to be at risk, and analyze the causes of problems such as a large number of queries left behind and a low SDV ratio, and formulate solutions to bring the data entry into line with the data management progress report. We also communicate with the project team in a timely manner on the performance indicators of risks, and analyze the causes and solutions of problems such as large number of lingering challenges and low SDV ratio, so as to control the data entry risks at normal times, and avoid the accumulation of data problems that increase the pressure of the project team or lead to the postponement of the project timeline at the time of mid-period analysis or database lock;
2、Timely communications via regular meetings or emails to handle project related issues and to discuss data entry and challenge response, reach a resolution in order to ensure that all research centers for the data issues dealt with in the same way. If there is a need to update the database or eCCI issues, PCDM should be recorded in the Issue Log in a timely manner in order to update the data files and database in a planned manner;
3、For projects with longer time and larger sample size, PCDM should make a prediction of the data entry volume according to the entry plan and program flow (SoA), and reasonably arrange the human resources to support the data cleanup work. PCDM can calculate the number of entry visits per month according to the visit arrangement and the DMP for data entry (visit-to-entry timeframe requirements) and make visit curves, in order to plan and adjust dataVerifiers. Regardless of timelines, it is recommended that challenges be processed at least once every two weeks and manual verification be completed once a month to avoid data issues going unverified and unchallenged for so long that the researcher is unable to track down the original record to update or clarify the data in question.
Regarding EDC system changes after the database has gone live, there are usually two situations that may lead to changing the database.
First, the system needs to be updated, and there is something that needs to be optimized in the previously setup system, such as the CRF design needs to be optimized (adding new units for combined medications), and there is an error in the configuration of the logical verification;
Second, the program makes changes and its changes have an impact on the CRF. For system changes, PCDM should strictly control the change process, first of all, confirm with the project team the content of the change and the database change timeline, according to the system characteristics to assess whether there will be a need to suspend the use of the database change, if necessary, should be communicated in advance with the project team and the researcher, and in addition, PCDM needs to work with the database construction specialist (DBD, Database) to assess the impact of the change on the existing data to ensure that the original data are not damaged, and should try to avoid the need for a large number of re-entry due to database changes.In addition, PCDM needs to evaluate the impact of database change on the existing data with Database Designer (DBD) to ensure that the original data is not damaged, and should try to avoid the occurrence of database changes that lead to a large number of re-entry of data. PCDM should make a detailed record of the changes in the relevant documents, and carry out adequate testing and data migration comparison of the changed system.
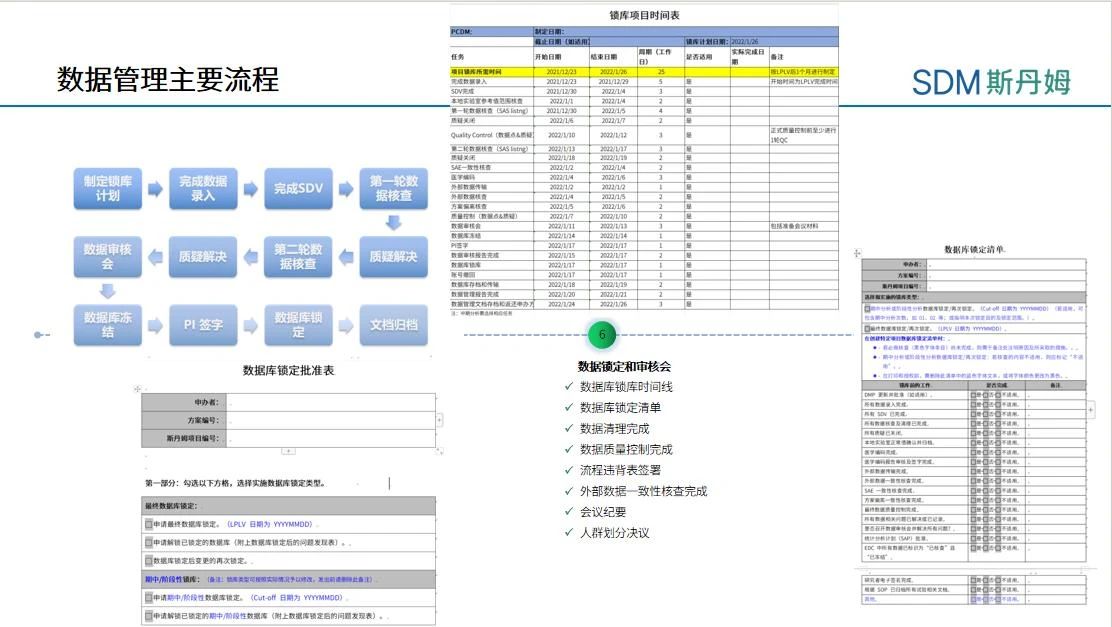
During the database lock phase, PCDM needs to develop the database lock schedule in advance and agree with the project team on the scheduling of the data cleansing timeline. The data management activities of database locking are interlocked, and each round of data cleansing needs to be carried out in the order of Source Data Verification (SDV), medical review and manual verification, and challenge processing, and generally three rounds of manual verification can be arranged before database locking to basically complete all the data cleansing work. Therefore, when the database is locked, PCDM not only needs to pay attention to the completion of work within the DM, but also needs to maintain close communication with the project team to ensure that the research center staff can complete data entry and challenge answering on time, the operation department can complete the SDV work on time, the medical monitoring team can complete the medical audit on time, the third-party vendor can return external data in a timely manner and solve the data problems, and the researcher (PI. Principal Investigator) completes the system e-signature on time, and the statistics department completes the finalization of the Statistical Analysis Plan (SAP, Statistical Analysis Plan). Therefore, during the database locking phase, PCDM should not only plan the timeline in advance, but also confirm the arrangement of vacation and back-up members with the project team and the research center in advance in case of vacation, in order to avoid delaying the project progress due to absence of personnel. During the database locking period, PCDM should also inform the project team of the completion of the timeline and the potential risk affecting the final locking intensively, and communicate with the project team on a daily basis if necessary.
In summary, there are four key verbs that PCDMs should grasp if they want to manage the project schedule:
Learn - carefully study the entire clinical trial protocol to understand important project nodes;
Communicate - engage in timely, two-way communication with the project team to understand project-related progress and keep the project team informed of data activity progress;
Planning and adjusting - planning ahead and adjusting data management efforts in real time based on project needs.
Only then can you better support the team and advance the overall project progress from a data work perspective.
References:
[1]: Guide to the Project Management Body of Knowledge (PMBOK® Guide), Sixth Edition
This session focuses on Stakeholder Engagement: developing strategies for collaborative project decision-making and execution.
The updated draft guidance involves a focus on clinical trial design, regulatory considerations, and whether these trials can demonstrate that the drugs can maintain weight loss as determined by BMI.
Let's take a look back at the previous two installments of the Clinical Data Management “PM” series: Scope Management, which clarifies the scope of responsibilities of all parties involved in a clinical trial, and Project Resource Management, which focuses on the utilization of company and personal resources to accomplish data management tasks. In this installment, we will focus on the most important part of project management - project schedule management - to share the timeline planning and progress follow-up of data management activities, so as to efficiently complete the data management work under the premise of ensuring the data quality and reaching the important milestones of the project.
In the last session, we learned about project scope management for data management work, identifying the scope of responsibilities for data cleansing and data management activities. After defining the scope of the data management work, in this session, we will learn how to mobilize the resources within the scope of work to carry out the data management work more efficiently and with higher quality.
Shanghai SDM Vaccine Data Management Department, in collaboration with the International Project Department, has launched a series of training sessions on "Application of Project Management Knowledge in Data Management." The Application of Project Management Knowledge in Data Management Work contains eight modules, including Project Integration Management, Project Scope Management, Project Progress Management, Project Quality Management, Project Resource Management, Project Communication Management, Project Risk Management, and Project Stakeholder Management, etc. It mainly refers to the theoretical knowledge of the Guide to the Project Management Body of Knowledge (PMBOK Guide) and combines the content of the data management work and practical experience of the project.
Want to quickly penetrate the Chinese, American and European pharmaceutical markets? Registering for communication exchanges is the key!
SDM PV team detects drug safety risks.
SDM Vaccine Experts Share Roadmap to Avoid Clinical Trial Pitfalls.
this article outlines essential documentation preparation and strategic considerations for conducting pre-IND communication meetings with CDE, ensuring effective regulatory alignment and adequate guidance.
Global Vaccine Solutions via Multidimensional Strategies.
July 23, 2025 GSK disclosed that the FDA has postponed PDUFA date of the Blenrep® (belantamab mafodotin) combination therapy BLA. The agency established a new action date of October 23, 2025 for completion of BLA review.
SDM Bioservices has successfully established a hybrid immuno-capture LC-MS/MS platform for the simultaneous quantification of ADC total antibody, conjugated antibody, conjugated drug, and free small-molecule payload. This approach significantly reduces reliance on specific antibody reagents, enables rapid method development and validation, and supports high-throughput sample analysis—thereby accelerating project timelines and advancing drug development efforts.
Premier Li Qiang has signed a State Council decree, promulgating the "Regulations on the Administration of Clinical Research and Translation of Novel Biomedical Technologies." This important regulation was adopted at the State Council executive meeting on September 12, 2025, and will take effect on May 1, 2026. This establishes a comprehensive legal framework for China's oversight of novel biomedical technologies throughout the entire chain from research to application.
Get in touch with SDM experts for your questions or comments and a member of our team will get back to you directly.
Let's Start a Conversation