The 6th Vaccine Innovation Forum (VIF World 2025) was held in Shanghai from March 20-22. Dr. Yuwen, SVP and Global CBO of SDM, chaired the Vaccine Clinical Development & Global Collaboration forum, presented Global Vaccine Registration Strategy and Key Considerations.
Dr. Yuwen, bringing 35 years of expertise spanning the FDA, global pharmaceutical firms, and CROs in Europe, the US and China, analyzed regulatory approval trends in China, the US, and Europe, evaluated global R&D progress alongside emerging developments, proposing strategic recommendations aligned with regulatory standards and global vaccine registration needs.

Dr. Yuwen citing Our World in Data, summarized vaccines' life-saving role through historical milestones like smallpox eradication and polio control. He highlighted systemic shifts in the vaccine industry – notably uneven global distribution – and called for strengthened international collaboration.

Dr. Yuwen then contrasted regulatory frameworks of the FDA (U.S.), EMA (EU), and NMPA (China), examining legal frameworks, approval bodies, special pathways, and data standards to outline region-specific procedures and critical requirements.
He emphasized key vaccine technology platforms, highlighting their mechanisms, applications, and case studies. Emphasizing RNA technology’s revolutionary role in COVID-19 for rapid adaptation to pathogens, and detailed its development process—from antigen design to delivery methods and AI integration. He concluded that vaccine R&D is advancing toward precision, diversification, and efficiency.
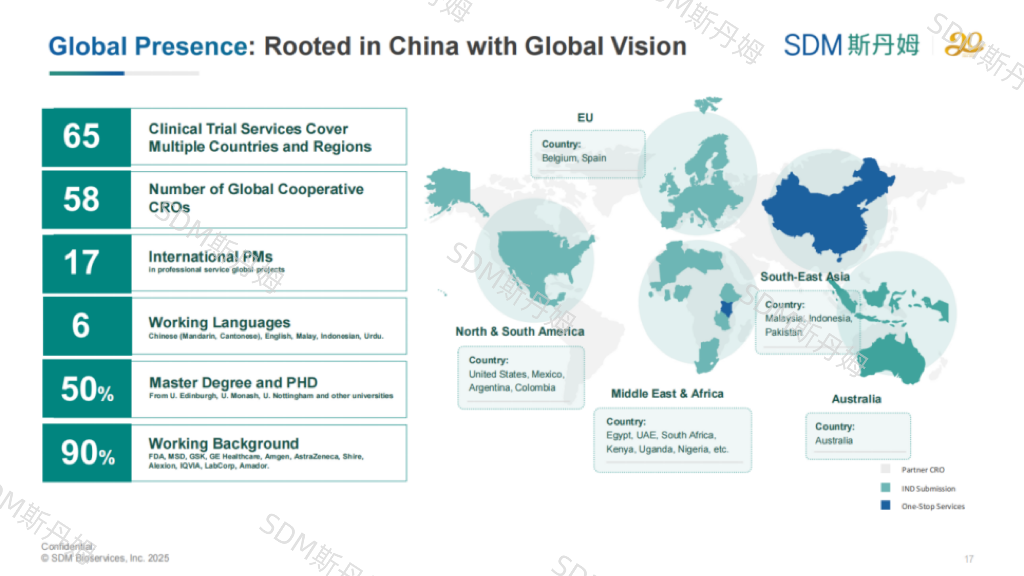
Dr. Yuwen concluded with strategic recommendations for global vaccine development: advancing technological innovation (including adjuvant system upgrades) and enhancing globalization efforts, with actionable steps for pre-IND preparation and post-IND submission.
The session offered vaccine professionals key insights into global R&D trends and strategies, sparking strong engagement at the conference. Attendees praised its actionable technical guidance and forward-looking vision.
Contact SDM and Dr Yuwen for tailored global drug registration solutions at any time.
This session focuses on Stakeholder Engagement: developing strategies for collaborative project decision-making and execution.
The updated draft guidance involves a focus on clinical trial design, regulatory considerations, and whether these trials can demonstrate that the drugs can maintain weight loss as determined by BMI.
Let's take a look back at the previous two installments of the Clinical Data Management “PM” series: Scope Management, which clarifies the scope of responsibilities of all parties involved in a clinical trial, and Project Resource Management, which focuses on the utilization of company and personal resources to accomplish data management tasks. In this installment, we will focus on the most important part of project management - project schedule management - to share the timeline planning and progress follow-up of data management activities, so as to efficiently complete the data management work under the premise of ensuring the data quality and reaching the important milestones of the project.
In the last session, we learned about project scope management for data management work, identifying the scope of responsibilities for data cleansing and data management activities. After defining the scope of the data management work, in this session, we will learn how to mobilize the resources within the scope of work to carry out the data management work more efficiently and with higher quality.
Shanghai SDM Vaccine Data Management Department, in collaboration with the International Project Department, has launched a series of training sessions on "Application of Project Management Knowledge in Data Management." The Application of Project Management Knowledge in Data Management Work contains eight modules, including Project Integration Management, Project Scope Management, Project Progress Management, Project Quality Management, Project Resource Management, Project Communication Management, Project Risk Management, and Project Stakeholder Management, etc. It mainly refers to the theoretical knowledge of the Guide to the Project Management Body of Knowledge (PMBOK Guide) and combines the content of the data management work and practical experience of the project.
Want to quickly penetrate the Chinese, American and European pharmaceutical markets? Registering for communication exchanges is the key!
SDM PV team detects drug safety risks.
SDM Vaccine Experts Share Roadmap to Avoid Clinical Trial Pitfalls.
this article outlines essential documentation preparation and strategic considerations for conducting pre-IND communication meetings with CDE, ensuring effective regulatory alignment and adequate guidance.
Global Vaccine Solutions via Multidimensional Strategies.
July 23, 2025 GSK disclosed that the FDA has postponed PDUFA date of the Blenrep® (belantamab mafodotin) combination therapy BLA. The agency established a new action date of October 23, 2025 for completion of BLA review.
SDM Bioservices has successfully established a hybrid immuno-capture LC-MS/MS platform for the simultaneous quantification of ADC total antibody, conjugated antibody, conjugated drug, and free small-molecule payload. This approach significantly reduces reliance on specific antibody reagents, enables rapid method development and validation, and supports high-throughput sample analysis—thereby accelerating project timelines and advancing drug development efforts.
Premier Li Qiang has signed a State Council decree, promulgating the "Regulations on the Administration of Clinical Research and Translation of Novel Biomedical Technologies." This important regulation was adopted at the State Council executive meeting on September 12, 2025, and will take effect on May 1, 2026. This establishes a comprehensive legal framework for China's oversight of novel biomedical technologies throughout the entire chain from research to application.
Get in touch with SDM experts for your questions or comments and a member of our team will get back to you directly.
Let's Start a Conversation