In recent years, domestic clinical trial registration has grown rapidly, with clinical data gaining prominence as a key translational outcome in research. Database construction and validation are crucial for ensuring data completeness and accuracy in clinical studies.
Driven by innovative drug development needs and the pursuit of time and cost efficiency, complex clinical trial designs are increasingly common. Examples include integrated early-phase SAD/MAD studies with Food Effect (FE), Drug-Drug Interaction (DDI), and Mass Balance (MB) components; oncology trials combining dose escalation and expansion; Decentralized Clinical Trials (DCT); and the merging of real-world and post-marketing data. These advanced designs demand robust database construction and integration, making efficient clinical database development a critical factor in improving research quality.
The clinical database architecture primarily relies on an EDC system, with core components being page design, logical validation, and access control (particularly blinding). Page layouts are structured around the Case Report Form (CRF), a critical trial element that ensures data completeness and study credibility. Effective CRF design aligns with trial objectives to collect research-relevant data, directly supporting clinical trial success.
CRF designs vary across clinical trials due to multiple factors: the absence of standardized templates adaptable to all study designs, conflicting priorities among team members or departments, and limitations of database platforms requiring system-specific adjustments. To optimize resource reuse, existing templates and standards should be prioritized where possible:
1. Variable Uniformity: Align variable names/coding with controlled terminology.
2. Option Consistency: Standardize option order (e.g., "Yes" before "No") and expressions (e.g., "Unknown/Not Done/Not Applicable").
3. Format Harmonization: Define formats for dates, decimals, fixed units.
4. Guideline Systematization: Optimize eCRF instructions via online tools for end-to-end consistency.
Standardized design improves data entry efficiency, quality, and reduces training and analysis complexity. Leverage global standards like CDISC/CDASH to streamline data collection and enhance interoperability.
CDASH Methodology for Data Acquisition System Design
CDASH establishes clinical data standards and CRF content guidelines for new drug trials, including common variable pools, with a recommended design process:
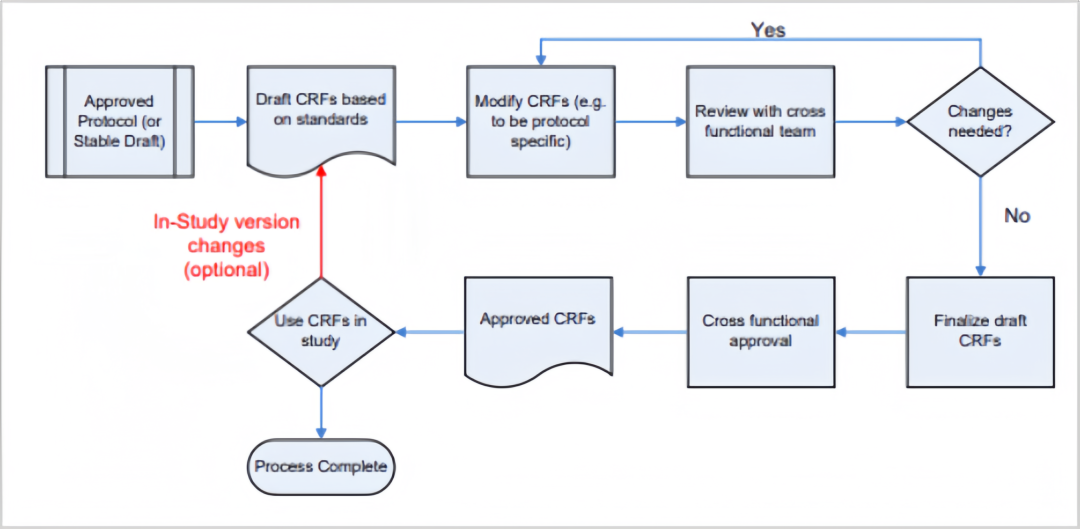
As can be seen from the above figure, CRF design is a collaborative and strictly controlled process requiring input from all clinical research stakeholders to review, refine, and approve the forms. The drafting phase begins only after finalizing the study protocol, with special attention to key requirements during this stage.
1. Data streamlining principle: Collect only essential variables for analysis to avoid redundancy. Excessive data increases costs/time while compromising quality, and raises ethical concerns (e.g., unnecessary PK sampling may impact subjects).
2. Analysis-driven principle: Align data collection with study protocol and statistical plans. Collect all required variables using statistically sound methods based on predefined analytical objectives.
3. Standardization: Adhere to CDASH/CDISC standards for data fields, minimizing free text requiring coding/extraction for analysis.
4. User-centered design: Implement intuitive field labeling and guidance to eliminate CRF ambiguities.
Leverage CDASH template libraries for CRF optimization
CDASH delivers prebuilt templates for core clinical data points, such as: Adverse Events(AE)、Comments(CO)、Prior and Concomitant Medications(CM)、Demographics(DM)、ECG Test Results(EG)、Exposure(EX)、Inclusion/Exclusion Criteria Not Met(IE)、Laboratory Test Results(LB)、Medical History(MH)、Physical Examination(PE)、Procedures(PR)、Subject Characteristics(SC)、Substance Use(SU)、Vital Signs(VS), etc. Here are two examples, though the study does not cover all listed fields.
1. The Adverse Events page lists routinely collected variables, such as: HR=Highly Recommended,R/C=Required/Conditional,O=Optional。
- AETERM-Adverse Event Name (HR)
- AESTDAT-Adverse event start date (HR)
- AESTTIM-Adverse event start time (R/C)
- AEENDAT-Adverse event end date (HR)
- AEENTIM-Adverse event end time (R/C)
- AESEV-Severity level (R/C)/AETOXGR-Toxicity level (R/C)
- AEREL-relationship to test drug (HR)
- AEACN-Actions taken with the test drug (R/C)
- AEOUT-Adverse event outcome (R/C)
- AEDIS-whether to withdraw from the trial because of this AE (O)
- AESER-Serious adverse event (R/C)
- AESDTH-Deadly (R/C)
- AESLIFE-Life-threatening (R/C)
- ASSHOSP-Hospitalization or prolonged hospitalization (R/C)
- AESDISAB-Causes permanent or significant disability/loss of function (R/C)
- AESCONG-Congenital anomaly or birth defect (R/C)
- AESMIE-Other medically significant event (R/C)
2. The Hypoglycemic Events page (Endocrine Program) lists potential variables to collect, such as:
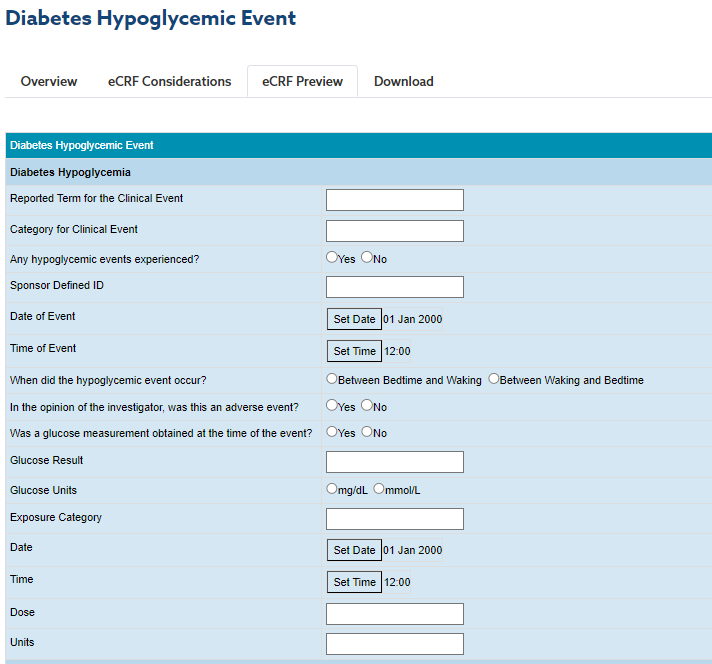
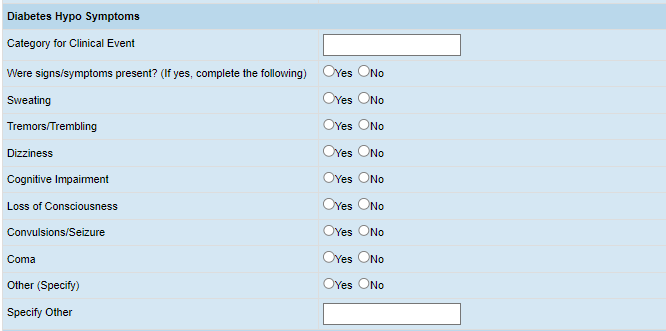
(CDISC, Tools, Knowledge Base, eCRF Portal)
When designing eCRFs from CRFs, it is critical to balance EDC system characteristics: While high reusability of fields/forms accelerates database setup, tightly linked modules make later modifications error-prone and laborious (like "pulling one hair affects the whole body"); conversely, low reusability isolates changes to individual forms but increases initial build time and duplicates fields. Thus, optimizing reusability versus efficiency requires aligning with project-specific needs.
The EDC system's logical validation ensures data accuracy, reduces data cleaning time, and simplifies statistical analysis. Key considerations for eCRF design and validation setup include:
1. Use standardized field names in eCRFs to streamline logical verification and minimize errors.
2. Modular design: Decouple collectable data elements (e.g., separate date/time fields) to optimize system logic and maintenance workflows.
3. Implement logical verification by avoiding wildcards and precisely referencing fields with specific identifiers (e.g., visit, form, field, record number).
4. Multi-record log verification is often more complex and error-prone than direct field configuration; always check system capabilities (e.g., dynamic row support) beforehand.
Standardizing CRF design and EDC setup—including logical verification—streamlines research processes. While adaptable designs lack universal solutions, prioritizing standardization, practical application of knowledge, and continuous learning transforms theory into action. These foundational ‘root system’ principles simplify complexity, enabling extraction of valuable data from information overload to ultimately advance human benefit.
This session focuses on Stakeholder Engagement: developing strategies for collaborative project decision-making and execution.
The updated draft guidance involves a focus on clinical trial design, regulatory considerations, and whether these trials can demonstrate that the drugs can maintain weight loss as determined by BMI.
Let's take a look back at the previous two installments of the Clinical Data Management “PM” series: Scope Management, which clarifies the scope of responsibilities of all parties involved in a clinical trial, and Project Resource Management, which focuses on the utilization of company and personal resources to accomplish data management tasks. In this installment, we will focus on the most important part of project management - project schedule management - to share the timeline planning and progress follow-up of data management activities, so as to efficiently complete the data management work under the premise of ensuring the data quality and reaching the important milestones of the project.
In the last session, we learned about project scope management for data management work, identifying the scope of responsibilities for data cleansing and data management activities. After defining the scope of the data management work, in this session, we will learn how to mobilize the resources within the scope of work to carry out the data management work more efficiently and with higher quality.
Shanghai SDM Vaccine Data Management Department, in collaboration with the International Project Department, has launched a series of training sessions on "Application of Project Management Knowledge in Data Management." The Application of Project Management Knowledge in Data Management Work contains eight modules, including Project Integration Management, Project Scope Management, Project Progress Management, Project Quality Management, Project Resource Management, Project Communication Management, Project Risk Management, and Project Stakeholder Management, etc. It mainly refers to the theoretical knowledge of the Guide to the Project Management Body of Knowledge (PMBOK Guide) and combines the content of the data management work and practical experience of the project.
Want to quickly penetrate the Chinese, American and European pharmaceutical markets? Registering for communication exchanges is the key!
SDM PV team detects drug safety risks.
SDM Vaccine Experts Share Roadmap to Avoid Clinical Trial Pitfalls.
this article outlines essential documentation preparation and strategic considerations for conducting pre-IND communication meetings with CDE, ensuring effective regulatory alignment and adequate guidance.
Global Vaccine Solutions via Multidimensional Strategies.
July 23, 2025 GSK disclosed that the FDA has postponed PDUFA date of the Blenrep® (belantamab mafodotin) combination therapy BLA. The agency established a new action date of October 23, 2025 for completion of BLA review.
SDM Bioservices has successfully established a hybrid immuno-capture LC-MS/MS platform for the simultaneous quantification of ADC total antibody, conjugated antibody, conjugated drug, and free small-molecule payload. This approach significantly reduces reliance on specific antibody reagents, enables rapid method development and validation, and supports high-throughput sample analysis—thereby accelerating project timelines and advancing drug development efforts.
Premier Li Qiang has signed a State Council decree, promulgating the "Regulations on the Administration of Clinical Research and Translation of Novel Biomedical Technologies." This important regulation was adopted at the State Council executive meeting on September 12, 2025, and will take effect on May 1, 2026. This establishes a comprehensive legal framework for China's oversight of novel biomedical technologies throughout the entire chain from research to application.
Get in touch with SDM experts for your questions or comments and a member of our team will get back to you directly.
Let's Start a Conversation