In the last session, we learned the scope of project management of data management, identifying the scope of responsibilities for data cleansing and data management activities. This session, we will learn how to mobilize the resources within the scope of work to carry out the data management work more efficiently and with higher quality after defining the scope of the data management work.
Project resource management involves the processes of identifying, acquiring, and managing the resources needed to successfully complete a project. These processes help to ensure that the project manager and the project team use the right resources in the right place at the right time; the resources that can be utilized in a project are mainly physical and human resources [1]. Physical resources involved in the data management process often include lessons learned and knowledge base; human resources often include members of the project team.
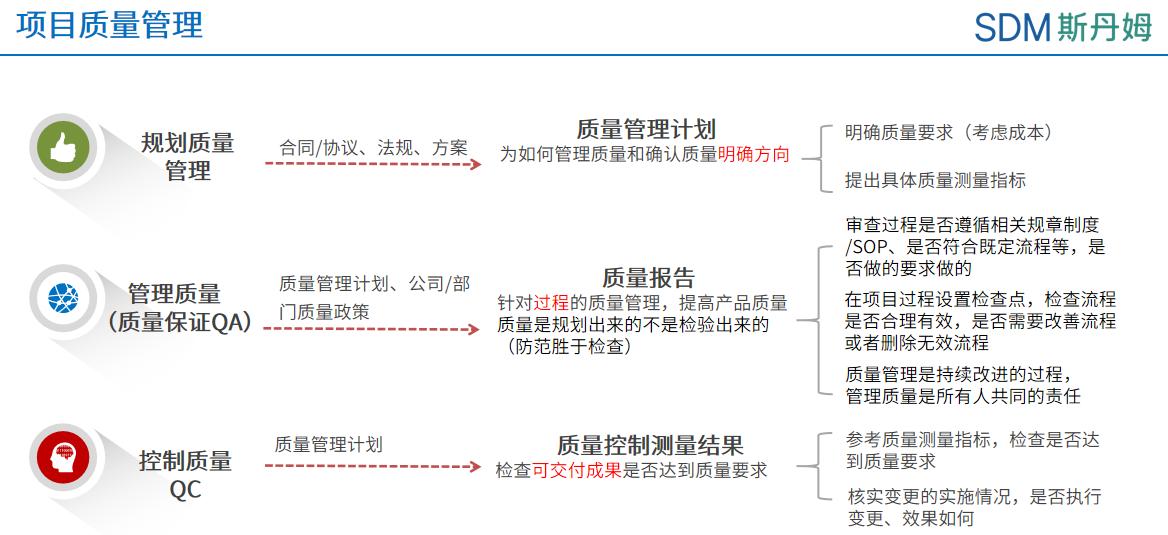
The process of project resource management is divided into six main processes: planning resource management, estimating activity resources, acquiring resources, building the team, managing the team, and controlling the resources [1]. The primary data manager is the manager of the team in the data management work and is responsible for building and managing an efficient team in all phases of data management such as initiation, cleansing, and locking the library. In the project startup phase, it is necessary to plan the resources for the data management work, allocate human resources and build the data team according to the expected workload of the project. In the data cleansing and locking phase, the main data manager also needs to dynamically adjust human resources according to the workload of different phases of the project in order to better manage the team and resources.
Resources in data management efforts
The primary data manager PCDM needs to make optimum use of the available resources of the company in carrying out the data management process. The resources related to data management can be broadly categorized into 3 major groups: human resources, documentation, experience and tools. The categorization of resources for data management may vary slightly from company to company, but the overall classification is similar.
Human Resources
The data team of a project usually consists of 1 PCDM, 1 DBD, and several CDMs. At the early stage of project initiation, the PCDM needs to consider a number of factors, such as the project's initiation timeline, therapeutic area, subject sample size, protocol complexity, etc., and apply for human resources from the line manager to build the data team, and the PCDM can inform the needs of the building team in terms of the personnel's work schedule, previous experience and competence. Upon receipt of PCDM's application for human resources, the line manager needs to consider the arrangement from the individual situation and the overall situation of the department, not only taking into account the workload undertaken by the personnel and the conflict of the project timeline, but also from the departmental level, considering that the workload and the difficulty of the project undertaken by the personnel should be relatively balanced, which will help the personal development and the improvement of the department's overall executive power. For CDM's work arrangement, PCDM needs to reasonably assign document writing, testing, data cleaning, medical coding, locking libraries and other related work according to the project timeline and the past experience of the team members, firstly to ensure the high quality delivery of the project, and at the same time, to consider the accumulation of skills and personal growth of CDM.
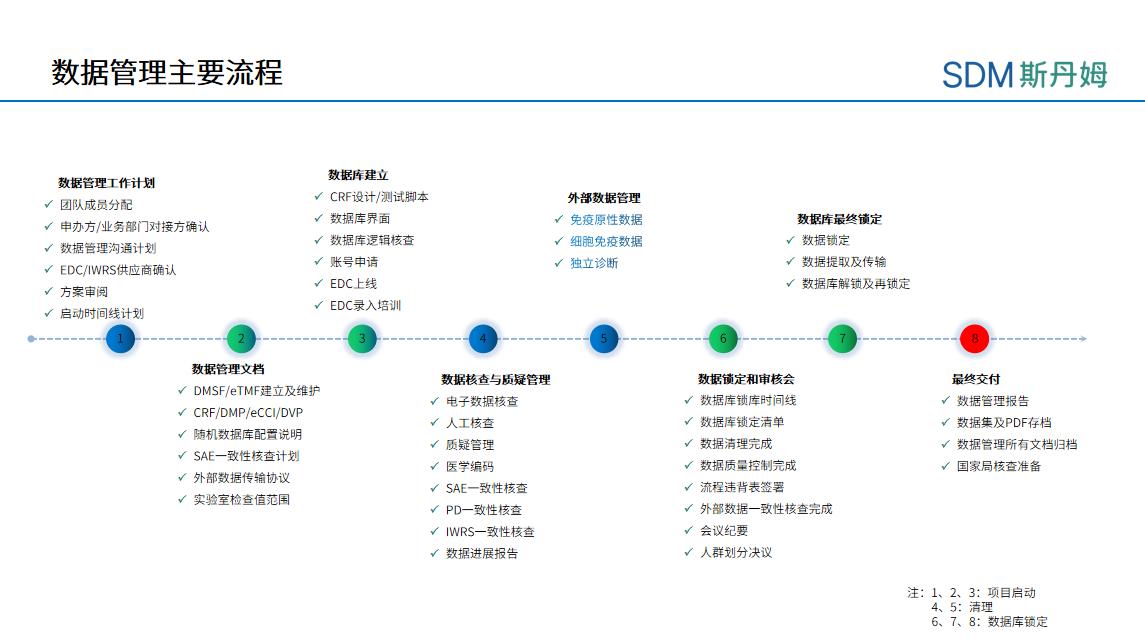
In the process of building a team, the data department needs to maintain tracking sheets of team members' work assignments and individual experience capabilities to more accurately understand each member's strengths and weaknesses in order to better coordinate human resources to deliver better project quality. In terms of project assignments and personnel experience, consider maintaining three tool sheets, Project Data Management Staff Assignment, DM Technical Assessment, and EDC Experience Collection. The Project Data Management Personnel Assignment is used to understand the past and current work schedule of project members, such as project volume, project timeline conflicts, blind/non-blind roles, etc. The DM Technical Assessment is used to assess department members' experience in various data management tasks at five levels: inexperienced, beginner, experienced, familiar, and mastery to assess the full process of data management activities from project initiation to lockup. The EDC Experience Collection is used to understand DBD's experience with different EDC repositories and programming experiences. The use of these tools facilitates the continuous improvement of the team's efficiency and the quality of project delivery.
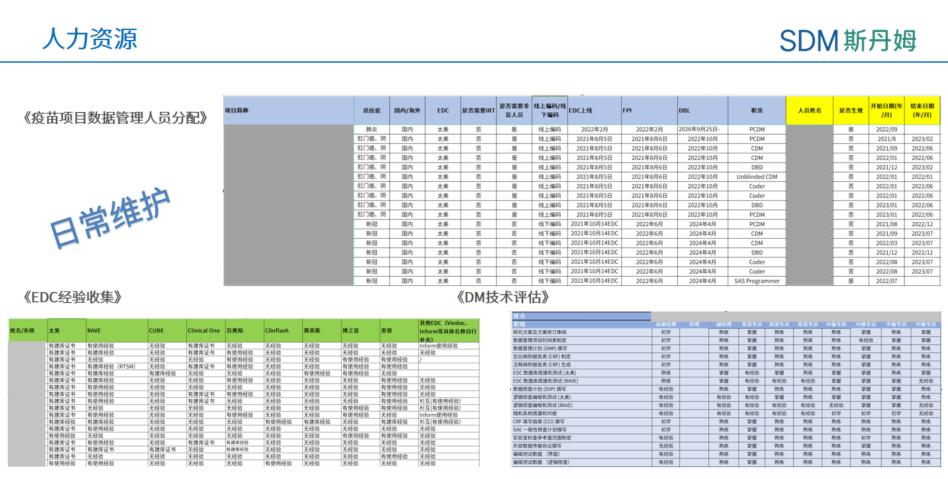
Documents related to Human Resource Management of Shanghai SDM Vaccine Data Management Department
In addition, the data management team needs to build a system of SME (Subject Matter Expert) in different DM knowledge domains in order to maintain DM knowledge in a more targeted way, such as build, startup, coding, data cleaning/locking, performance, non-blind, random, and QC. SMEs in each area collect data-related questions from within the DM, other business units, or sponsors, and provide feedback and answers to the questions, initiate discussions when necessary, and update SOPs or standard documents when a resolution is reached by the data team.
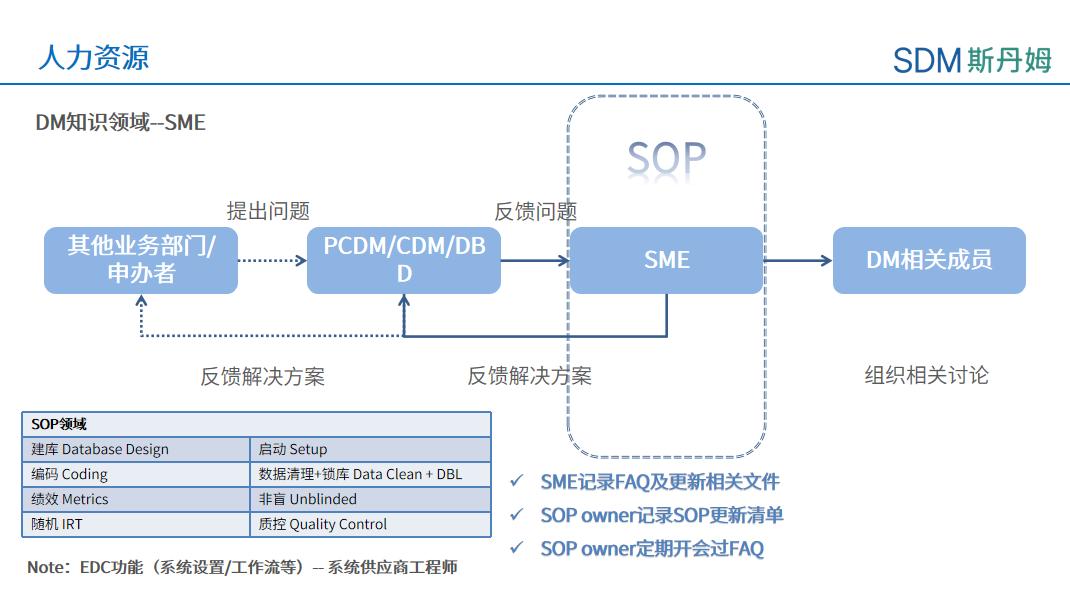
SME System Management Process of Shanghai SDM Vaccine Data Management Department
PCDM should also be able to utilize external resources. When it may involve other specialized knowledge, such as medicine, operations, etc., the PCDM can seek help from other business team members to solve cross-discipline related data problems; when encountering EDC system problems, the PCDM can consult with EDC system vendors; and can also discuss with DMs of other companies who have related experience to obtain the optimal solution to the problem.
Documentation and experience
While completing the daily projects, the data management team should also reserve the whole process of documentation for data management work, and constantly update the accumulated experience to the reserve knowledge base in the process of project practice, in order to continuously optimize the workflow and improve the efficiency and quality of data management work.The documents and experiences that can be referred to in the process of clinical data management can be divided into four parts: SOP documents, non-SOP documents, training + experience sharing, and external resources.
SOP documents can contain processes, working guidelines, templates, forms and other contents. If there is a need for overseas projects, consideration should be given to establishing bilingual versions of SOPs to facilitate the rapid development and application of overseas projects.
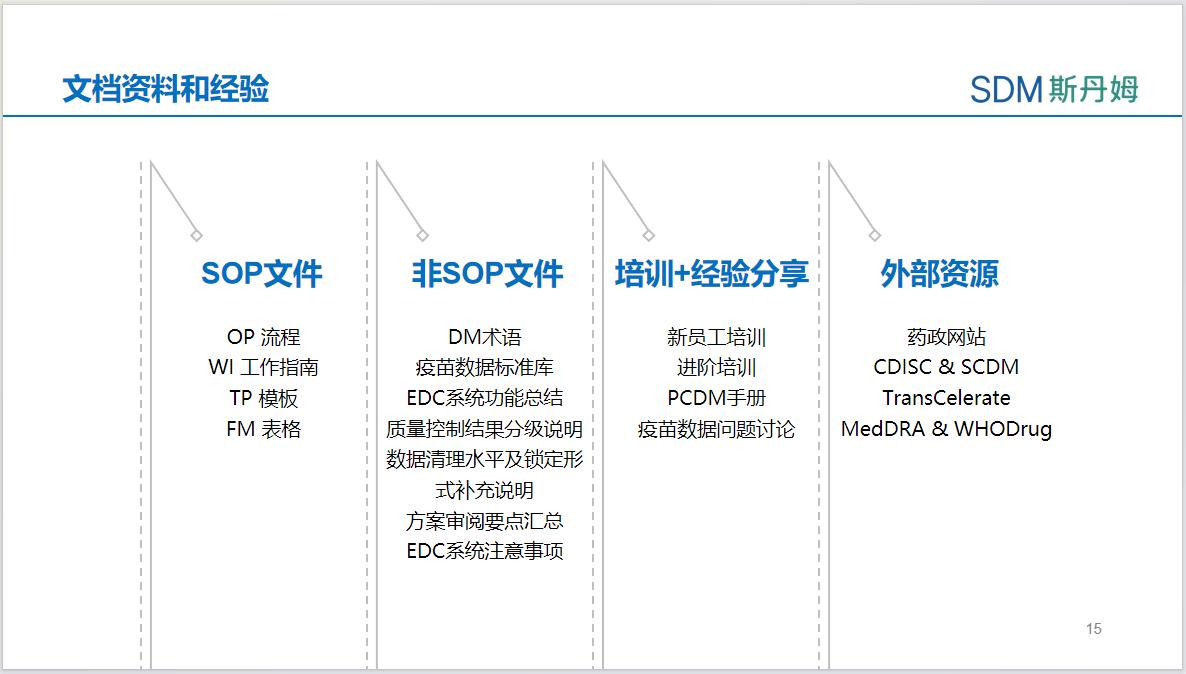
SOP documents can contain processes, working guidelines, templates, forms and other contents. If there is a need for overseas projects, consideration should be given to establishing bilingual versions of SOPs to facilitate the rapid development and application of overseas projects.
SOP documents can be used to guide the overall workflow and standardize data-related documents, but in order to cope with the complex and changing requirements of clinical trials and reasonably avoid the compliance risk brought by SOP violation, there is also a need to have non-SOP specification documents to stipulate and guide the detailed parts of data management work and supplement the data management SOP.
For example: "Quality Control Results Grading Instructions" can describe the quality grading of process management, document design and data cleansing in quality control to more specifically assess the quality of project data; "Data Cleansing Levels and Locking Forms Supplementary Instructions" can provide suggestions on data cleansing levels and locking forms for different data submission requirements in order to simplify and standardize the data management process; "Summary of Key Points for Program Review" can guide the data management team in interpreting the data management work details.The "Summary of Program Review Points" can guide the data management team in interpreting each part of the program in order to better design documents such as CRFs, data verification plans, etc. The "EDC System Considerations" can summarize the functions of each EDC system such as the workflow settings for the data management team's reference.
Training can enhance the skill level of project members so that they can perform their job duties, solve complex problems, and anticipate and avoid project risks.Based on the extensive documentation in the field of data management and the frequent need to apply cross-departmental domain knowledge, the data management team should have a dedicated training group to organize intra-departmental and cross-departmental training and experience sharing. New employee training should include the whole process of data management, and after consolidating the foundation, the data management team should provide advanced training to help improve data management skills, such as advanced training in medical coding and drug coding, medical training related to therapeutic areas, the application of standardized documents, and offline verification of difficult issues, etc., as well as soft skills training in time management, prioritization, cross-departmental communication, and project management to help team members improve their communication skills.The PCDM can also provide training and coaching at the project level, or apply for training resources from the department.
The collection and sharing of experience can help the data management team optimize processes and improve the execution of team members. The collection and summarization of experience can be carried out at the project level and departmental level respectively. At the project level, PCDM can maintain a project-level Issue Log, which records and organizes relevant team members to discuss data issues of the project. In order to summarize and share data experience and knowledge at the departmental level, PCDM can feedback common issues to SMEs in the data department, who will organize colleagues within or across departments to discuss data issues, and then update SOPs or non-SOPs related documents after reaching a resolution, and carry out internal and external trainings to strengthen understanding of the lessons learned.

Feedback Mechanism for Data Problems in Shanghai SDM Vaccine Data Management Department
In addition to internal resources, data management practitioners can also refer to the data management resources provided by domestic and foreign pharmaceutical authorities and non-profit organizations on their official websites, such as the CDE website on the guidelines for data submission and data management, the FDA website on data collection and data submission guidance documents, the CDISC website on the CDASH and other data standards, SCDM website on the GCDMP and other data management process webinars, etc. TransCelerate website also provides a wealth of clinical trial-related literature for practitioners to search and reference. The SCDM website provides webinars on GCDMP and other data management processes, etc. In addition, the TransCelerate website also provides a wealth of clinical trial-related literature for practitioners to search and refer to.
Tool resources
In the process of clinical data management, the development of tools is conducive to improving the efficiency of data management work, and commonly used data management tools such as online coding platforms, which can be used for MedDRA and other data management processes, are also available. The online coding platform can realize the automatic coding of MedDRA and WHODrug and the export of coding reports, which helps to improve the efficiency of offline coding, can avoid the errors caused by manual copying and pasting, and can ensure the consistency of the coding of different projects. Compare Tool can compare the old and new reports, realize the efficiency of manual data verification, and reduce the man-hours of reviewing the old data repeatedly; the construction of SFTP platform guarantees the security of data transmission; Metrics program can realize the automated generation of data reports and provide real-time and accurate progress of the project data to the sponsors, and so on.
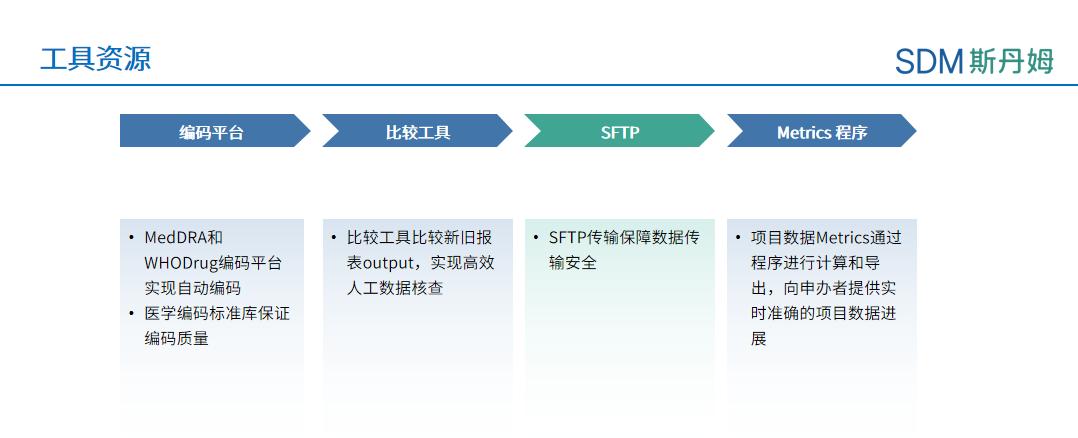
Shanghai SDM Vaccine Data Management Department Tool Resources
Project resource management is an important part of project management, and good resource management helps the project management team to optimize the use of resources, improve the management efficiency of the project, and reduce the cost and risk of the project. Dynamic project resource management adjustments in data management efforts will help project members grow and projects succeed.
References:
[1]: Guide to the Project Management Body of Knowledge (PMBOK® Guide), Sixth Edition
[2]:https://www.cdisc.org/
[3]:https://scdm.org/
[4]:https://www.transceleratebiopharmainc.com/
[5]:https://www.cde.org.cn/zdyz/listpage/9cd8db3b7530c6fa0c86485e563f93c7
[6]:https://www.fda.gov/
This session focuses on Stakeholder Engagement: developing strategies for collaborative project decision-making and execution.
The updated draft guidance involves a focus on clinical trial design, regulatory considerations, and whether these trials can demonstrate that the drugs can maintain weight loss as determined by BMI.
Let's take a look back at the previous two installments of the Clinical Data Management “PM” series: Scope Management, which clarifies the scope of responsibilities of all parties involved in a clinical trial, and Project Resource Management, which focuses on the utilization of company and personal resources to accomplish data management tasks. In this installment, we will focus on the most important part of project management - project schedule management - to share the timeline planning and progress follow-up of data management activities, so as to efficiently complete the data management work under the premise of ensuring the data quality and reaching the important milestones of the project.
In the last session, we learned about project scope management for data management work, identifying the scope of responsibilities for data cleansing and data management activities. After defining the scope of the data management work, in this session, we will learn how to mobilize the resources within the scope of work to carry out the data management work more efficiently and with higher quality.
Shanghai SDM Vaccine Data Management Department, in collaboration with the International Project Department, has launched a series of training sessions on "Application of Project Management Knowledge in Data Management." The Application of Project Management Knowledge in Data Management Work contains eight modules, including Project Integration Management, Project Scope Management, Project Progress Management, Project Quality Management, Project Resource Management, Project Communication Management, Project Risk Management, and Project Stakeholder Management, etc. It mainly refers to the theoretical knowledge of the Guide to the Project Management Body of Knowledge (PMBOK Guide) and combines the content of the data management work and practical experience of the project.
Want to quickly penetrate the Chinese, American and European pharmaceutical markets? Registering for communication exchanges is the key!
SDM PV team detects drug safety risks.
SDM Vaccine Experts Share Roadmap to Avoid Clinical Trial Pitfalls.
this article outlines essential documentation preparation and strategic considerations for conducting pre-IND communication meetings with CDE, ensuring effective regulatory alignment and adequate guidance.
Global Vaccine Solutions via Multidimensional Strategies.
July 23, 2025 GSK disclosed that the FDA has postponed PDUFA date of the Blenrep® (belantamab mafodotin) combination therapy BLA. The agency established a new action date of October 23, 2025 for completion of BLA review.
SDM Bioservices has successfully established a hybrid immuno-capture LC-MS/MS platform for the simultaneous quantification of ADC total antibody, conjugated antibody, conjugated drug, and free small-molecule payload. This approach significantly reduces reliance on specific antibody reagents, enables rapid method development and validation, and supports high-throughput sample analysis—thereby accelerating project timelines and advancing drug development efforts.
Premier Li Qiang has signed a State Council decree, promulgating the "Regulations on the Administration of Clinical Research and Translation of Novel Biomedical Technologies." This important regulation was adopted at the State Council executive meeting on September 12, 2025, and will take effect on May 1, 2026. This establishes a comprehensive legal framework for China's oversight of novel biomedical technologies throughout the entire chain from research to application.
Authored by the SDM Bioanalysis Team, this article delves into the principles, delivery systems and advantages of mRNA technology, provides a comprehensive overview of its applications and clinical breakthroughs in three major fields—infectious disease prevention, cancer therapy and protein replacement therapy—and elaborates on the key bioanalytical methodologies.
Get in touch with SDM experts for your questions or comments and a member of our team will get back to you directly.
Let's Start a Conversation